Credit for images: An Introduction to Medicinal Chemistry (Graham L. Patrick)
Oral Drug Absorption and Amines
- Many oral drugs which are efficiently absorbed contain amines.
- Amines develop an equilibrium between charged and uncharged forms in the slightly basic intestines and the slightly acidic blood. Based on the Henderson-Hasselbalch equation (below), amines are approximately 50% ionized in a pH of 6-8.

- When the amine is charged, it has good water solubility and can form ionic interactions with the drug’s target.
- When the amine is uncharged, it can pass through membranes and be absorbed more easily.

Oral Drug Absorption Guidelines
- If a drug is too hydrophilic, it will not pass through the cell membranes of the gut wall.
- If a drug is too hydrophobic, it will dissolve and get stuck in fat globules and be poorly soluble in the rest of the gut.
- Lipinski’s rule of five provides general guidelines for characteristics that may allow good absorption of a drug.
- Molecular weight less than 500
- Less than or equal to 5 hydrogen bond donor groups
- Less than or equal to 10 hydrogen bond acceptor groups
- Calculated logP value of less than 5 (logP is a measure of hydrophobicity)
- An alternate system involves using the polar surface area of a drug. In this system, it was found that more freely rotatable bonds often indicates less oral bioavailability. To have good oral bioavailability according to this system a drug should possess:
- Either a polar surface area of ≤140 Å and ≤10 rotatable bonds (excluding rotatable bonds of simple groups like hydroxyl and methyl that don’t greatly affect the shape of the molecule)
- Or ≤12 total hydrogen bond donors and acceptors and ≤10 rotatable bonds (excluding rotatable bonds of simple groups like hydroxyl and methyl that don’t greatly affect the shape of the molecule)
- Some highly polar oral drugs can break these guidelines by being taken up by membrane transporter proteins. This is often the case when the drug has a similar structure to the usual substrate of the transporter.
- Some highly polar oral drugs with molecular weights of less than ~200 can be absorbed by traveling between the cells on the gut wall.
- Some highly polar oral drugs can be absorbed by transcytosis across the gut wall.
- Some oral drugs are deliberately designed to be highly polar so as to keep them in the gut. This is exemplified by antibiotics for gastrointestinal infections.
Drug Distribution through the Body
- Although it only takes ~1 minute for a drug to be evenly distributed through the bloodstream (as that is approximately how long the blood takes to cycle through the body), various barriers decrease the efficiency with which the drug is distributed to the rest of the body.
- Most capillaries have 90-150 Å gaps between endothelial cells. This allows most drugs to diffuse into tissues, but blocks plasma proteins.
- Blood vessels in the blood-brain barrier do not have gaps between endothelial cells. In addition, astrocytes coat the blood vessels with fatty layers, preventing polar drugs from entering the brain.
- Sometimes polar drugs can enter the brain by transcytosis or by being transported on carrier proteins.
- Often, the blood-brain barrier is beneficial because CNS side effects from many drugs (which are not intended for the brain) are prevented.
- Sometimes drugs are bound by plasma proteins and cannot escape capillaries.
- If a drug has an intracellular target, it must somehow enter the cell to act.
- Highly hydrophobic drugs are often absorbed into fat tissue and removed from the blood supply (especially in obese patients). This can prevent them from reaching their site of action.
- Many drugs can easily pass the placental barrier since this barrier is permeable a variety of nutrients and waste products.
- Fat soluble drugs pass through most easily. They can reach the same levels between fetal blood and maternal blood and may be toxic to the fetus.
- Once a baby is born, he/she may retain high levels of the drug in his/her system. Developmentally, the baby is less able to remove and detoxify the drug than an adult, so this can be dangerous.
- If two drugs both are bound by serum proteins like albumin, taking both at the same time results in some of each being displaced from the serum proteins via binding competition. This can result in higher levels of the drugs being released into the body.
Drug Metabolism
- Drug metabolism usually inactivates a drug, but sometimes it can activate a prodrug or other times result in a metabolite with side effects.
- Polar drugs are easily excreted by the kidneys, but nonpolar drugs are more difficult to remove.
- Nonpolar drugs tend to be metabolized via attachment of polar groups to their susceptible moieties.
- Phase I drug metabolism involves non-specific enzymes such as cytochrome P450s (mainly in the liver) attaching polar groups to drug molecules or removing moieties which block polar groups that already exist in the drug molecule (such as via demethylating a methyl ether).
- Most phase I reactions are oxidations, reductions, or hydrolysis reactions.
- N-methyl groups, aromatic rings, terminal carbons of alkyl groups, and others are particularly susceptible to oxidation.
- Nitro, azo, and carbonyl groups are particularly susceptible to reduction.
- Amides and esters are particularly susceptible to hydrolysis.
- Phase II drug metabolism involves conjugating the newly attached polar groups (or preexisting polar groups) on the drug to another polar molecule. This also mainly occurs in the liver.
- For a drug to approved, all of its metabolites (and their stereochemical conformations) in humans must be characterized for biological activity.
Phase I Metabolism and Cytochrome P450s
- There are at least 33 types of cytochrome P450 in humans. They are grouped into families CYP1-CYP4. Subfamilies are designated by a letter and the specific enzyme is designated by another number (for example CYP3A4).
- Cytochrome P450s contain a heme group. They break molecular oxygen and attach one of the oxygen atoms to the drug and convert the other oxygen atom to water. Thus, they are part of the monooxygenase class of enzymes.
- More exposed drug moieties are more susceptible to cytochrome P450s.
- Methyl groups are often easily oxidized to make alcohols, longer alkyl groups are usually oxidized on the terminal carbon.
- The most exposed parts of aliphatic rings are most likely to be oxidized.
- Activated carbon atoms are more easily oxidized by cytochrome P450s.
- Allylic or benzylic carbons are actually even more easily oxidized than exposed carbons.
- Hydroxylation of α-carbons which are adjacent to a heteroatom (another type of activated carbon) results in an unstable intermediate which is hydrolyzed. This hydrolysis causes dealkylation of amines, ethers, and other groups.
- Tertiary amines are more reactive than secondary amines to dealkylation because of their greater basicity (so long as their alkyl groups are not too sterically hindering).
- Tertiary amines are oxidized to N-oxides, while other amines are also oxidized to N-oxides but then undergo subsequent reactions to become hydroxylamines and other compounds.
- Primary amines extending from aromatic rings can be oxidized to nitro groups, which can undergo other reactions that produce toxic alkylating agents.
- Cytochrome P450s can oxidize sp2 and sp carbons in alkenes, alkynes, and aromatic rings to form epoxides.
- The epoxides are then deactivated by epoxide hydrolase to form diols.
- Sometimes epoxides evade the epoxide hydrolase and react with DNA, proteins, and other molecules thus causing toxic effects.
- Oxidized aromatic rings with epoxides may either interact with epoxide hydrolase, undergo rearrangements to form phenols, or undergo other reactions.
- Thiols can be oxidized to disulphides.
- As with any enzyme, cytochrome P450s sometimes catalyze reactions in the opposite direction, thus facilitating reductive processes.



Phase I Metabolism and Other Enzymes
- The liver ER contains flavin monooxygenases, a class of enzymes which includes some cytochrome P450s as well as other proteins. These enzymes tend to oxidize nucleophilic nitrogen, sulphur, and phosphorous but not carbon.
- Monoamine oxidases deaminate catecholamines and oxidize certain drugs.
- Alcohol dehydrogenases produce aldehydes which are then oxidized to carboxylic acids by aldehyde dehydrogenases.
- Some reductive reactions also occur in phase I metabolism, though they are less common. Cytochrome P450s (as mentioned) or other enzymes can be involved.
- Esterases and peptidases are also involved in phase I metabolism. They hydrolyze esters and amides (generally amides are hydrolyzed more slowly than esters). Electron withdrawing groups on esters and amides can increase the rate of their hydrolysis.

Phase II Metabolism
- Phase II metabolism involves the conjugation of polar groups (often added in phase I metabolism) on drugs to other molecules.
- The most common type of phase II reaction is conjugation to glucuronic acid.
- Phenols, alcohols, hydroxylamines, and carboxylic acids react with UDFP-glucuronate to form O-glucuronides.
- Sulphonamides, amines, amides, and thiols react with UDFP-glucuronate to form N– or S-glucuronides.
- Activated carbons (like α carbons) next to carbonyls react with UDFP-glucuronate to form C-glucuronides.
- These reactions are catalyzed by glucuronyltransferases.


- Another (less common) type of phase II reaction is sulphate conjugation.
- This occurs mainly with phenols, alcohols, arylamines, and N-hydroxy compounds.
- These reactions are catalyzed by sulphotransferases which use the cofactor 3’-phosphoadenosine 5’-phosphosulphate as the source of sulphate.
- Primary alcohols which are sulphonated often become toxic reactive alkylating agents. The other sulphonated groups are generally stable.

- Drugs with carboxylic acid groups can be conjugated to amino acids via the formation of a peptide bond.The most common amino acid used for this purpose in primates is glutamine.
- Drugs with carboxylic acid groups can be conjugated to cholesterols by transesterification reactions.
- Occasionally, alcohol groups on drugs may be conjugated to fatty acids via an ester linkage.
- Electrophilic groups like epoxides, alkyl halides, sulphonates, disulphides, and radicals can react with the nucleophilic thiol of the tripeptide glutathione to form glutathione conjugates.
- These reactions are catalyzed by glutathione transferase.
- Glutathione conjugates can be converted to mercapturic acids.
- These reactions can be used to inactivate environmental toxins or electrophilic alkylating agents from phase I reactions.
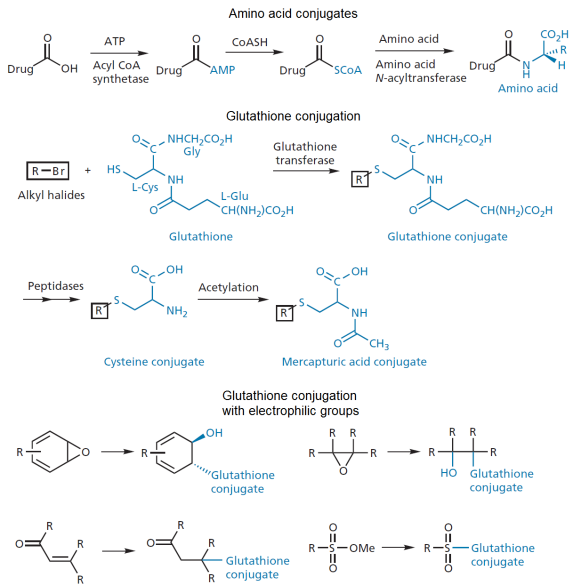
- A few conjugation reactions can actually decrease the polarity of the resultant compound.
- These are most commonly acetylations (using the cofactor acetyl CoA) and methylations (using the cofactor S-adenosyl methionine). Note that the exception here is that methylation of a pyridine nitrogen makes a polar quaternary ammonium salt.
- Acetylations usually occur on primary amines.
- Methylations usually occur on phenols, amines, and thiols.

Different Metabolic Stabilities
- Cytochrome P450 activities are extremely variable between patients.
- Substantial numbers of patients even lack certain cytochrome P450 isoforms. This can lead to dangerous buildup of some drugs in their bodies or a lack of activity of some prodrugs.
- Differences in drug metabolism are especially pronounced between populations, thus different countries often have different recommended doses.
- Pharmacogenomics may allow effective patient-specific drug regimens to be implemented based on genetic markers (personalized medicine).
- Cytochrome P450 activity can be affected by foods, drugs themselves, and other chemicals.
- Brussels sprouts and cigarette smoke enhance activity.
- Grapefruit juice inhibits activity, sometimes causing toxic buildup of drugs or prodrugs.
- Certain antibiotics can inhibit cytochrome P450s. This can result in unexpected toxicity if another drug is being taken simultaneously.
- Some drugs stimulate cytochrome P450s. If such a drug is taken along with another drug, then the activity of the other drug may be lower than expected.
- New drugs are usually tested for effects on cytochrome P450s.
- Terminology: hard drugs are less susceptible to metabolism while soft drugs or antedrugs are more susceptible to metabolism and are designed to be metabolized in a controlled manner so that they can accomplish their function and then be eliminated.
- Oral drugs must pass through the liver immediately when they enter the blood and thus will be subjected to liver enzymes. This is called the first pass effect. Other methods of delivery allow the drug to move around the body before passing through the liver, with some molecules never entering the liver due to diffusion into the tissues.
Drug Excretion
- Gaseous drugs are excreted by diffusing into the lungs and being exhaled (the reverse of how they are administered).
- Some oral drugs may be incorporated into bile by passing into bile ducts in the liver and then be returned to the intestines, reducing the amount of drug that reaches the rest of the body (the amount distributed is less than the amount absorbed).
- About 10-15% of some drugs can be lost through the skin via sweat. Saliva and breast milk can also contribute to drug elimination, but the amount lost is much less. The main concern with breast milk is that an infant may then consume some of the drug.
- Drugs are principally excreted by the kidneys. They enter via the renal artery which branches into arterioles and feeds into nephrons.
- Small molecule drugs filter through the glomerulus. Afterwards, they pass through the proximal and distal tubules, but are partially reabsorbed by surrounding capillaries.
- Aquaporins in the proximal and distal tubules remove water, concentrating the drug in the tubules and leading to faster diffusion out of them.
- Hydrophobic drugs are easily reabsorbed from the proximal and distal tubules, while polar drugs remain trapped within the tubules before being excreted. In this way, drug metabolism is able to speed the excretion of xenobiotics even if they start as hydrophobic molecules.
- Some drugs are actively transported from the blood vessels into the kidneys.

Choosing a Bioassay for Drug Testing
- To avoid wasting resources on ineffective drugs, generally in-vitro bioassays are conducted first, then animal models, and then clinical trials. This helps control for increasing numbers of variables as the more complex systems are utilized.
- In-vitro bioassays can be carried out on isolated enzymes (which were produced in recombinant bacteria or yeast), bacteria or other microorganisms (for antimicrobials), or on mammalian or yeast cells expressing a target protein of interest. Some more complex in-vitro bioassays include those which use hepatocyte tissue culture to expose drugs to cytochrome P450s and those which use artificial membranes to model drug absorption across the blood-brain barrier.
- In-vivo bioassays are animal models (and later, clinical trials). Many animal models are humanized, they are recombinant and express certain human proteins. Animal models are expensive, slow, legally elaborate, and sometimes have different results than clinical trials. Nevertheless, they are an essential part of the drug development process.
- Test validity: if a drug is being used to treat a condition for which most models are inadequate, such as in the cases of many mental illnesses, it is important (yet difficult) to select a method of testing. In-vitro models involving receptors central to the disease process are often chosen to start, but these can be problematic due to poor understanding of the role of the receptor in the disease. One may even skip animal trials for some of these types of situations.
High-Throughput Screening
- Automated screening of thousands of compounds, often with dozens of different types of biochemical tests.
- A method of measurement, such as the displacement of radiolabeled ligands from receptors, cell growth, color change, etc., is necessary.
- Generally, positive hits are compounds with activity at concentrations of 1 nM to 30 μM.
- False positive hits are a major issue.
- One major source of false positive hits are promiscuous inhibitors. These compounds inhibit many targets with little selectivity.
- Promiscuous inhibitors are often aggregates of multiple compounds which adsorb target proteins to their surfaces.
- Promiscuous inhibitors tend to be more common in solutions with mixtures of compounds.
- Adding a detergent to the test solution will usually break up promiscuous inhibitor aggregates.
- Another source of false positive hits are reactive compounds which form covalent bonds with their targets. Although some drugs do behave this way, in high-throughput screening, such compounds are usually not target-specific and thus are not useful.
Nuclear Magnetic Resonance Screening
- NMR induces certain atomic nuclei to enter an excited spin state.
- When the nuclei return to ground state, they release energy that can be measured. The pattern of energy release can characterize specific molecules.
- The amount of time taken for nuclei to return to the ground state is called the relaxation time.
- The relaxation time for small molecules like drugs tends to be much longer than macromolecules like proteins and drug-protein complexes.
- To screen drugs by NMR, the following steps are generally performed.
- The NMR spectrum of the drug on its own is measured.
- The target protein (or other macromolecule) is treated with the drug.
- The NMR spectrum of the (potential) drug-protein complex is measured, but with a delay so that only free drug molecules will be detected if they are present. (Because the free drug molecules have long relaxation times relative to the drug-protein complexes). If the drug molecules have bound to the target macromolecules, they will no longer be detectable in the sample.
- NMR holds an array of advantages and disadvantages.
- NMR can screen about 1,000 different compounds per day using a single machine.
- NMR can be used to determine if hits from high-throughput screening are false positives.
- NMR can identify weakly binding compounds (that may have otherwise been missed) which may then be modified to improve their affinity.
- NMR can identify the drug binding site region of the macromolecule.
- A major disadvantage is that NMR requires at least 200 mg of purified target protein for standard screening procedures.
Affinity Screening
- The target molecule is immobilized on a solid substrate.
- A mixture of potential drug compounds (such as from an extract) is exposed to the treated substrate.
- The mixture is removed and tested for activity elsewhere. If the mixture has lost its activity, it is likely due to active compounds being adsorbed onto the treated substrate.
- The active compounds can then be removed from the substrate (often by changing the pH) and tested.
- This is especially useful for identifying antibiotics.
Surface Plasmon Resonance
- Surface plasmon resonance employs a layered surface. The bottom layer is a glass plate. The next layers are a 50 nm gold film, a 100 nm dextran layer, and a flowing buffer solution on top (the buffer also enters the dextran).
- Embedded in the dextran layer are covalently immobilized ligand molecules.
- Monochromatic plane polarized light is shown at an angle of incidence (which is greater than the critical angle in order to produce total internal reflection) from below the layers.
- Despite the total internal reflection, if the proper angle is selected, a small component of the light called the evanescent wave will interact with the gold film’s free electrons (called plasmons) and deposit energy into them. This will cause a reduction in the measured intensity of the reflected light.
- If the refractive index of the buffer changes, then the angle that produces surface plasmon resonance will also change.
- The introduction of the target macromolecule into the buffer flow will result in a change of the buffer’s refractive index if the ligand binds the macromolecule. This can determine the targets to which a ligand might bind. It can also be used to determine binding equilibrium and rate constants.
- To test a novel drug compound, a known ligand for the macromolecule should be immobilized in the dextran and then both the drug and the target can be added to the buffer flow. In this way, if the drug binds the target, less target will be available to bind the known ligand, and the surface plasmon resonance angle will not change as much.

Scintillation Proximity Assay
- The target macromolecule is covalently linked to beads that are coated with a scintillant (a substance which produces light when it absorbs ionizing radiation).
- A known ligand (of the target) radioactively labeled with iodine-125 is added to the solution. When it binds the target, the radiation makes the scintillant produce light.
- The drug molecule is added to the solution. If it binds the target, less of the labeled ligand will be able to bind the target and thus less light will be emitted.
Isothermal Titration Calorimetry
- Two glass cells are filled with buffer. One of them also contains a protein target in solution. Both cells are heated slightly to the same temperature.
- The drug molecule is added to the sample cell.
- If the drug binds the target, then energy will either be released or absorbed depending on whether the binding is exothermic or endothermic.
- Increases or decreases in the sample cell’s temperature relative to the reference cell indicate the binding of the drug to the target and the reaction’s thermodynamic properties.
- The temperature changes are measured as “spikes” which occur when the amount of power necessary to maintain a constant temperature in the cells is altered.
Virtual Screening
- Computer programs can determine if a compound is likely to bind a target.
- There is no guarantee that the virtual predictions will be correct, but they can “narrow the search” by identifying the best candidates from large libraries of structures. These candidates then can be tested experimentally.
Finding Lead Compounds from Natural Sources
- The identification of active compounds from natural sources is called pharmacognosy.
- Many active compounds are secondary metabolites.
- Active compounds are commonly obtained from a variety of organisms.
- Plants are a rich source of lead compounds. Unfortunately, few plants are well-studied and there are estimated to be numerous plant species which are completely unknown. In addition, many potentially useful plants are going extinct due to anthropogenic activity (especially rainforest destruction).
- Microorganisms (mainly fungi and bacteria) often produce many antimicrobial compounds. In addition, some microorganisms produce compounds with other therapeutically useful activities.
- Marine organisms such as coral, sponges, fish, and marine microorganisms are a relatively unexplored source of valuable compounds.
- Animals sometimes produce useful compounds.
- Toxins from arthropods, vertebrate animals, and microorganisms often have potent biological activity and bind specifically to target macromolecules. Analogs of some toxins can be highly effective drugs.
- Active compounds can also be identified by examining the medical folklore of certain cultures. Although usually ineffective, effective by placebo, or harmful, a few substances used by such cultures contain active compounds which may be developed into drugs.
Finding Lead Compounds from Synthetic Compound Libraries
- Numerous synthetic compounds are prepared by pharmaceutical companies and can be tested for activity. However, many of these are just variations of the same backbone and thus are less likely to have novel activities.
- Sometimes pharmaceutical companies will purchase compounds from university laboratories. In many of these cases, such compounds are not intended to have a pharmaceutical function and are part of other types of research, yet they still have potential.
- Intermediate compounds in drug synthesis (and other syntheses) can be tested for biological activity.
Finding Lead Compounds from Existing Drugs
- Companies may modify their competitor’s drugs to create new versions for testing. Sometimes, these new drugs may achieve better activity than the originals (as in the case of penicillins).
- If an existing drug has a potentially useful side effect, then that drug may be modified to remove its original intended activity and enhance the side effect (selective optimization of side activities or SOSA). In rare cases, such drugs may even not require modification to be approved for an alternate purpose.
Find Lead Compounds from Natural Ligands, Substrates, and Related Structures
- Natural ligands for receptors can sometimes be modified to act as drugs.
- Existing biological agonists may be improved to create effective drugs.
- Creating an antagonist from an agonist sometimes can be achieved by adding extra binding groups.
- When the natural ligand for a receptor is unknown (an orphan receptor), research to uncover that ligand can be worthwhile for the purpose of opening a new avenue of drug design.
- Enzyme substrates can be modified to create effective inhibitors since they already bind to the enzyme. Also, because enzymes catalyze reactions in both directions, enzyme products can be used the same way.
- Natural allosteric regulators of receptors and enzymes can be modified to make drugs as well.
Structure-Activity Relationships
- After the structure of a lead compound is uncovered, the functional groups of the compound which contribute to its activity or stability in the body should be identified.
- By either modifying the structure of the lead compound to individually remove functional groups or by performing total syntheses to make versions of the compound individual functional groups, the importance of each group to activity of the drug can be tested.

Binding Role of Alcohols and Phenols
- The the hydroxyl group found in alcohols and phenols can act as a hydrogen bond donor or acceptor, contributing to binding interactions. These hydrogen bonds have directional preferences.

- Substituting a methyl ether or ester for the hydroxyl group produces analogs that can test for whether the hydroxyl group contributed to the drug’s activity. It is often relatively easy to accomplish these syntheses.
- The groups can thus no longer act as hydrogen bond donors.
- Although the analogs still would contain oxygens that could act as hydrogen bond acceptors, the steric hindrance caused by the bulkier groups reduces the degree to which this occurs.
- In the case of esters, there is also a resonance structure which decreases the ability of the more easily accessible oxygen (the alkoxy group oxygen) to act as a hydrogen bond acceptor. A positive formal charge is placed on this oxygen because some of its electrons move towards the central carbon in the resonance structure.

Binding Role of Aromatic Rings
- Because aromatic rings are planar and nonpolar, they are often involved in van der Waals or hydrophobic interactions with similarly flat and nonpolar regions of the binding site.

- Aromatic rings can also interact with quaternary ammonium ions at the binding site via induced dipole interactions.
- Using a cyclohexane ring (which is not planar) in place of an aromatic ring produces analogs that can test whether the aromatic ring contributed to the drug’s activity.
- The nonplanar cyclohexane ring is bulky (and thus can’t fit into small binding pockets) and its axial hydrogens keep the ring at a distance from the binding site.
- The methods used to convert aromatic rings to cyclohexane rings are usually not successful with lead compounds, so to accomplish this transformation, total synthesis is most often used.
Binding Role of Alkenes
- Alkenes are similar to aromatic rings in that they interact with the binding site via van der Waals or hydrophobic interactions.
- Saturation (reduction) of alkenes to alkanes increases their bulk due to the additional hydrogens and prevents as close of approach to the binding site. This can create analogs to test whether the alkene contributes to the drug’s activity.
- Alkenes are often easier to reduce than aromatic rings, so analogs can sometimes be prepared directly from the lead compound.

Binding Role of Carbonyls
- Ketones are planar groups that can use their oxygen as a hydrogen bond acceptor. Two possible hydrogen bonds can be formed this way because of the two lone pairs on the oxygens.

- Ketones also can initiate dipole-dipole interactions because of their dipole moment.
- Ketones can be reduced to alcohols, changing the geometry from planar to tetrahedral and thus weakening hydrogen bonding and dipole-dipole activity. This can create analogs to test whether the ketone contributes to the drug’s activity.
- If the hydroxyl oxygen still may be acting as a hydrogen bond acceptor, then ether or ester groups should be attached described for alcohols and phenols.

- Aldehydes are less common in drugs due to their reactivity, but if they are present, similar strategies to those of the ketones can be used for testing their role in binding.
Binding Role of Amines
- Amines can use their lone pair as a hydrogen bond acceptor. The hydrogens of primary and secondary amines (but not tertiary or aromatic amines) can each act as hydrogen bond donors.
- If the amine is protonated, it cannot accept hydrogen bonds, but its hydrogen bond donating activity is stronger. In addition, ionic interactions with the target can occur.

- To create analogs to test whether the amine contributes to the drug’s activity, the amine can be replaced by an amide group.
- It is relatively easy to change a primary or secondary amine to an amide without needing to perform total synthesis.
- Tertiary amines can often be changed to amides if one of their R groups is a methyl group. This is accomplished by first removing the methyl group with vinyloxycarbonylchloride (VOC-Cl). This technique was used with morphine.
