PDF Version: Notes on Upconversion Nanoparticles – Logan Thrasher Collins
Background and Overview
- Photoluminescence spectroscopy (also called fluorescence spectroscopy) uses electromagnetic radiation to excite electrons in various materials. Photons are emitted from the materials as the electrons return to lower energy states. In biology and biomedicine, photoluminescence spectroscopy provides a noninvasive tool which allows for visualization of biological processes over a wide range of scales (subcellular to macroscale).
- Many biological applications of fluorescence spectroscopy require exogenous contrast agents like organic dyes, fluorescent proteins, quantum dots, and metal complexes. But such agents often possess limitations including (i) low signal-to-background ratio as a result of tissue autofluorescence and scattering when short wavelengths are used, (ii) low penetration depth when UV or visible light excitation is used as well as when UV or visible light are emitted, (iii) potential DNA damage from short wavelengths like UV, and (iv) potential toxicity of contrast agents containing heavy metals like cadmium and lead.
- Near-infrared (NIR) wavelengths provide a potentially superior alternative to other types of excitation for many situations. Biological tissue exhibits “optical transparency” in the NIR range of 700-1100 nm, allowing for deeper tissue penetration. In addition, NIR usually causes less autofluorescence and scattering. Some other alternatives exist, but these generally require expensive ultrashort pulsed lasers, so they are often less practical than NIR techniques.
- Lanthanide-doped upconversion nanoparticles (UCNPs) absorb two or more low-energy photons and emit a single photon with a higher energy. In this way, the long wavelengths of NIR can be converted to shorter wavelength radiation like visible light and UV upon emission.
Nanochemistry of UCNPs
- In lanthanide-doped UCNPs, trivalent lanthanide cations (elements with atomic numbers in the range of 57-71 which possess a +3 charge) are embedded in a chosen dielectric lattice that is 100 nm or less in diameter. Dielectric lattices are crystalline solids which possess a net polarity due to the contributions of individual atoms within the lattice. This polarization can be quantified by an optically-measureable lattice dielectric constant.
- Using appropriate lanthanide dopants allows for wavelength-selective upconversion. NIR excitation wavelengths are converted to specific emission wavelengths. For instance, NIR can be selectively converted to blue light by some UCNPs and to ultraviolet by other UCNPs.
- When UCNPs are excited by NIR light, electrons in the trivalent lanthanide cations undergo 4f-4f orbital electronic transitions. Since the 5s and 5p shells provide an electronic shielding effect, the transitions are sharp rather than broad. The 4f-4f transitions which occur in UCNPs would ordinarily be forbidden by quantum mechanical laws, but interactions of the lanthanide ions with the crystal lattice of the nanoparticle allow “orbital mixing” which facilitates such transitions.
- The upconversion photoluminescence intensity of UCNPs exhibits a nonlinear dependence of the number of excitation photons required to induce photoluminescence from the nanoparticles. Here, the number of excitation photons is given by n, K is a material-specific constant, and P is the power of the laser (called a pump laser) used to excite the nanoparticles. It should be noted that a saturation effect occurs at high excitation energy densities, leading to a decrease in the apparent value of n.
![]()
- Upconversion quantum yield (UCQY) is defined as the number of photons emitted per photon absorbed. It is proportional to the ratio of the emitted upconversion light intensity to the absorbed light intensity. Using these relations and the equation for upconversion photoluminescence intensity, the equation below can be derived. Here, α is an absorption constant that is specific to the material and the excitation wavelength.
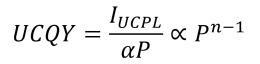
Upconversion Mechanisms
- There are five basic upconversion mechanisms including excited-state absorption, energy transfer upconversion, cooperative sensitization upconversion, cross relaxation, and photon avalanche.
- Excited-state absorption: multiple photons are absorbed by a single lanthanide ion, exciting electrons up a ladder-like series of energy levels. It should be noted that
 only certain lanthanides possess the proper characteristics for this mechanism to occur (Ho3+, Er3+, Nd3+, and Tm3+) when commercially-available diode lasers are used (which usually have incident wavelengths of around 975 or 808 nm). The relevant energy levels are called G, E1, and E2 (for ground state, excited state 1, and excited state 2). Electrons in the E1 excited state have long lifetimes before they return to the ground state G. As such, after an electron is excited to E1, it remains there for long enough that it can be promoted to E2 by another photon. When the electron decays from E2 all the way to G, this large change in energy causes a photon with a shorter wavelength to be emitted (shorter than the photons involved in the excitation).
only certain lanthanides possess the proper characteristics for this mechanism to occur (Ho3+, Er3+, Nd3+, and Tm3+) when commercially-available diode lasers are used (which usually have incident wavelengths of around 975 or 808 nm). The relevant energy levels are called G, E1, and E2 (for ground state, excited state 1, and excited state 2). Electrons in the E1 excited state have long lifetimes before they return to the ground state G. As such, after an electron is excited to E1, it remains there for long enough that it can be promoted to E2 by another photon. When the electron decays from E2 all the way to G, this large change in energy causes a photon with a shorter wavelength to be emitted (shorter than the photons involved in the excitation). - Energy transfer upconversion: a lanthanide ion called the sensitizer absorbs a photon and is excited to E1, then transfers its energy to an ion called the activator. The sensitizer then decays back to G. This occurs twice, causing the activator to be
 excited to E1 and then to E2. When the electron decays from E2 all the way to G, this large change in energy again causes a photon with a shorter wavelength to be emitted. Some of the most efficient UCNPs used for biomedical purposes operate by energy transfer upconversion and involve sensitizer/activator ion pairs of Yb3+/Tm3+, Yb3+/Er3+, or Yb3+/Ho3+ with excitation wavelengths of about 975 nm. The Yb3+ ion does not have an E2 energy level, it can only be excited from G to E1. This makes the upconversion process more efficient since the two energy levels of Yb3+ cannot cause cross-relaxations (a phenomenon which has a deleterious effect on upconversion). In addition to Yb3+-based UCNPs that work by energy transfer upconverion, some single-lanthanide UCNPs have also been developed. In these, the single type of lanthanide ion acts as a sensitizer and an activator.
excited to E1 and then to E2. When the electron decays from E2 all the way to G, this large change in energy again causes a photon with a shorter wavelength to be emitted. Some of the most efficient UCNPs used for biomedical purposes operate by energy transfer upconversion and involve sensitizer/activator ion pairs of Yb3+/Tm3+, Yb3+/Er3+, or Yb3+/Ho3+ with excitation wavelengths of about 975 nm. The Yb3+ ion does not have an E2 energy level, it can only be excited from G to E1. This makes the upconversion process more efficient since the two energy levels of Yb3+ cannot cause cross-relaxations (a phenomenon which has a deleterious effect on upconversion). In addition to Yb3+-based UCNPs that work by energy transfer upconverion, some single-lanthanide UCNPs have also been developed. In these, the single type of lanthanide ion acts as a sensitizer and an activator. - Cooperative sensitization upconversion: two ions of the same type absorb excitation photons and are excited to E1 states. Next, both ions simultaneously
 transfer energy to a third ion (which can be of a different type) and excite the third ion to its own E1 state. In this case, the E1 state of the third ion is twice as energetic as the E1 states of the other two ions. When the third ion returns to its ground state, it releases a photon with a shorter wavelength than the incident photons. Cooperative sensitization upconversion is much less efficient than the other mechanisms, but it may have some advantages for high-resolution imaging applications. The cooperative sensitization mechanism has been reported to occur with Yb3+/Tb3+, Yb3+/Eu3+, and Yb3+/Pr3+ ion pairs.
transfer energy to a third ion (which can be of a different type) and excite the third ion to its own E1 state. In this case, the E1 state of the third ion is twice as energetic as the E1 states of the other two ions. When the third ion returns to its ground state, it releases a photon with a shorter wavelength than the incident photons. Cooperative sensitization upconversion is much less efficient than the other mechanisms, but it may have some advantages for high-resolution imaging applications. The cooperative sensitization mechanism has been reported to occur with Yb3+/Tb3+, Yb3+/Eu3+, and Yb3+/Pr3+ ion pairs. - Cross relaxation: an ion is excited to E2 and then transfers its energy to a second ion as it returns to E1, exciting the second ion to E1. This mechanism depends on
 ion-ion interactions, so it is closely associated with dopant ion concentration. It can cause quenching at high concentrations, but tuning ion concentrations allows modulation of emitted light colors via other mechanisms. Note that cross relaxation on its own does not cause upconversion, but that it can influence upconversion events as other steps occur.
ion-ion interactions, so it is closely associated with dopant ion concentration. It can cause quenching at high concentrations, but tuning ion concentrations allows modulation of emitted light colors via other mechanisms. Note that cross relaxation on its own does not cause upconversion, but that it can influence upconversion events as other steps occur. - Photon avalanche: processes of excited-state absorption and cross-relaxation interact to create a positive feedback loop. Minimal upconversion occurs until a threshold of power input is passed, but the upconverted photoluminescence intensity vastly increases once the threshold has been exceeded. First, excited-state absorption causes an ion at E1 to transition into E2. Then this ion transfers its
 energy to a second ion, its electron goes down to E1, and the second ion’s electron is excited from G to E1 by cross relaxation. Last, the second ion transfers its energy to a third ion, resulting in two ions at the E1 state by the end of the loop. In this way, more and more ions are excited to the E1 state as the loop repeats (two, four, eight, etc.) Some of the ions in the E2 state (from the first step) will decay to G, causing upconverted photons to be released.
energy to a second ion, its electron goes down to E1, and the second ion’s electron is excited from G to E1 by cross relaxation. Last, the second ion transfers its energy to a third ion, resulting in two ions at the E1 state by the end of the loop. In this way, more and more ions are excited to the E1 state as the loop repeats (two, four, eight, etc.) Some of the ions in the E2 state (from the first step) will decay to G, causing upconverted photons to be released.
Reference: Chen, G., Qiu, H., Prasad, P. N., & Chen, X. (2014). Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chemical Reviews, 114(10), 5161–5214. http://doi.org/10.1021/cr400425h